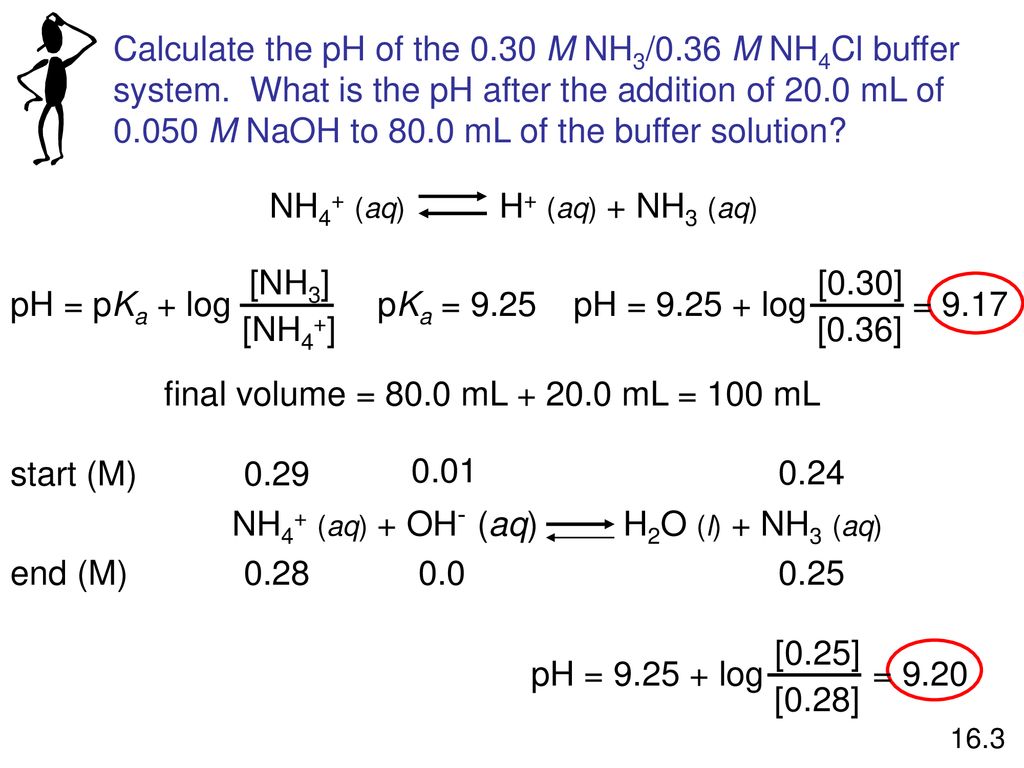
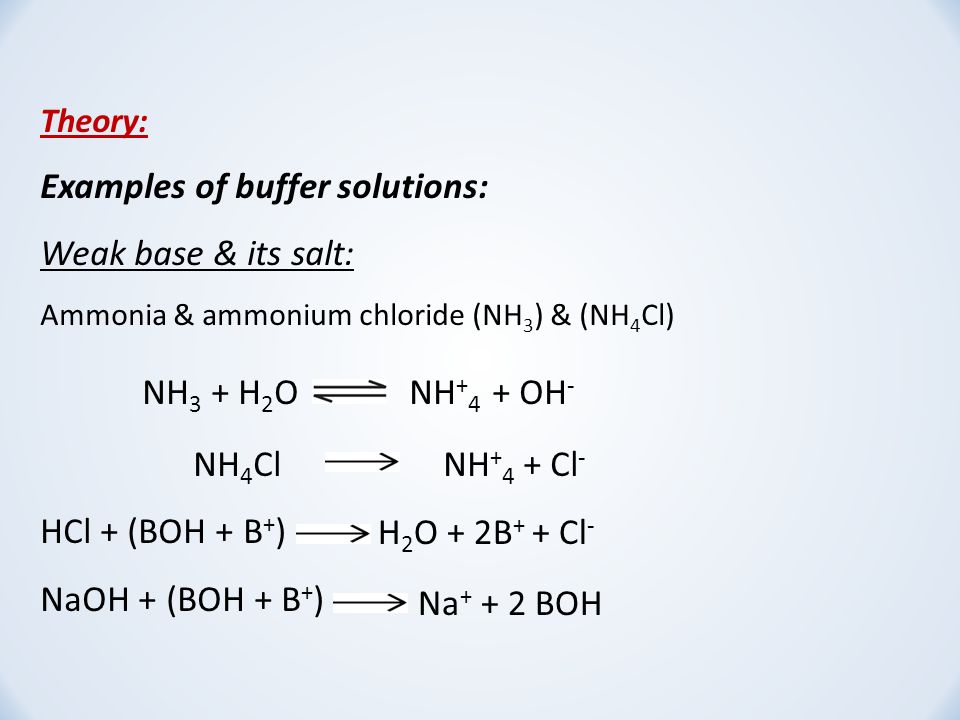
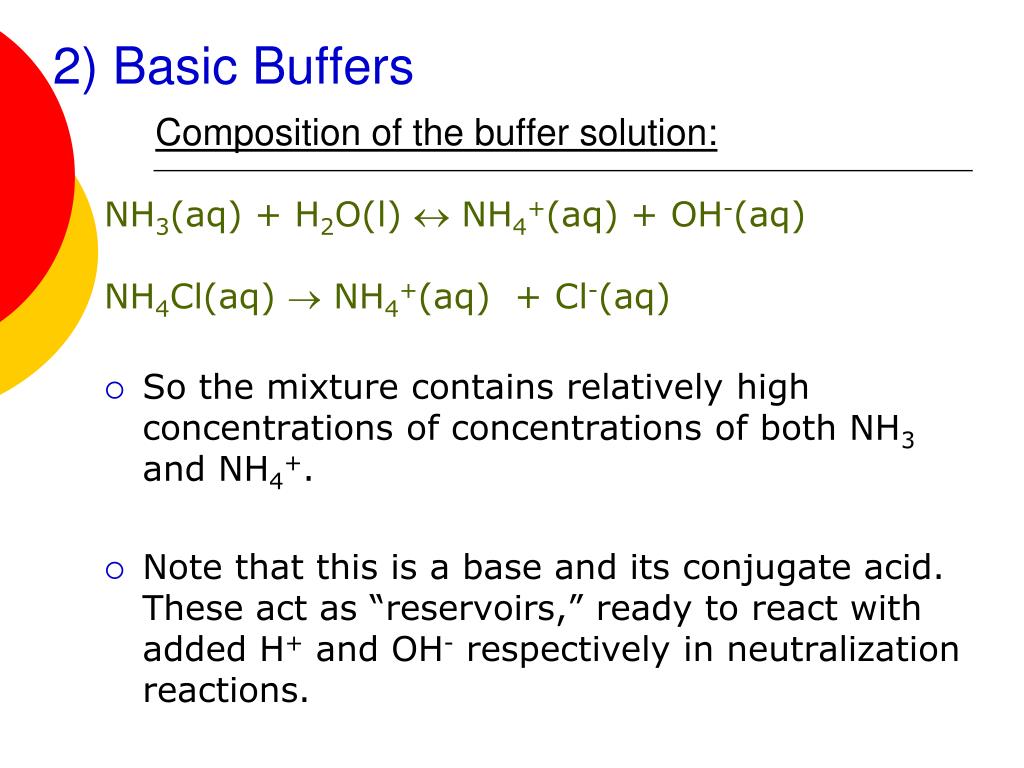
pH calculation of a buffer solution made from a weak base and its conjugate acid (salt form) - YouTube
Calculate the amount of NH3 and NH4Cl required to prepare a buffer solution of pH = 9 when total concentration of buffering - Sarthaks eConnect | Largest Online Education Community

OneClass: A)Calculate the pH of the 0.20 M NH3/0.24 M NH4Cl buffer. B)What is the pH of the buffer af...

Determination of 1 × 10 −5 M Co(III) with standard additions ((b), (c),... | Download Scientific Diagram